Introduction
Epidiolex is an FDA-approved medication that contains cannabidiol (CBD) as the active ingredient. It has been specifically developed to treat two severe forms of childhood epilepsy:
Lennox-Gastaut Syndrome (LGS) and Dravet Syndrome. This comprehensive guide aims to provide an overview of Epidiolex, its mechanism of action, recommended dosage, potential side effects, and its effectiveness in managing LGS and Dravet Syndrome in children.
Table of Contents
Understanding Lennox-Gastaut Syndrome (LGS)
- Definition and prevalence: Lennox-Gastaut Syndrome is a severe form of childhood epilepsy characterized by multiple seizure types and intellectual disability. It typically appears between the ages of 3 and 5. LGS is rare and affects approximately 1-5% of children with epilepsy.
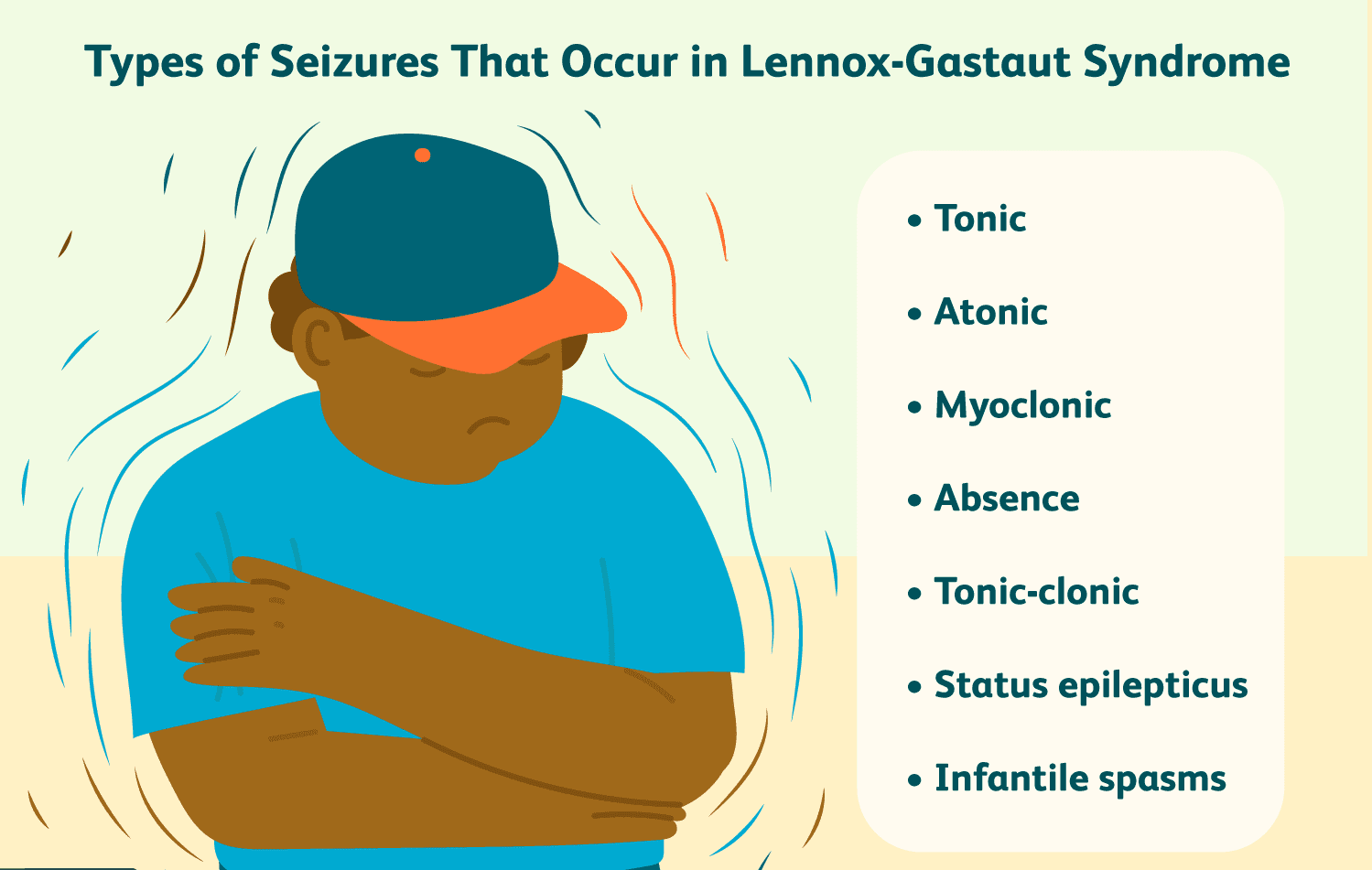
- Symptoms and characteristics: LGS is characterized by different types of seizures, including tonic seizures (stiffening of muscles), atonic seizures (sudden loss of muscle tone), and atypical absence seizures. Children with LGS often have developmental delays, cognitive impairment, and behavioral issues.
- Challenges in managing LGS: LGS is challenging to manage due to the resistance of seizures to many traditional anti-seizure medications. Children with LGS often have poor seizure control and experience frequent seizures, which can significantly impact their quality of life.
Understanding Dravet Syndrome
- Definition and prevalence: Dravet Syndrome, also known as Severe Myoclonic Epilepsy of Infancy (SMEI), is a rare genetic disorder that starts in infancy. It affects approximately 1 in 15,000 individuals.
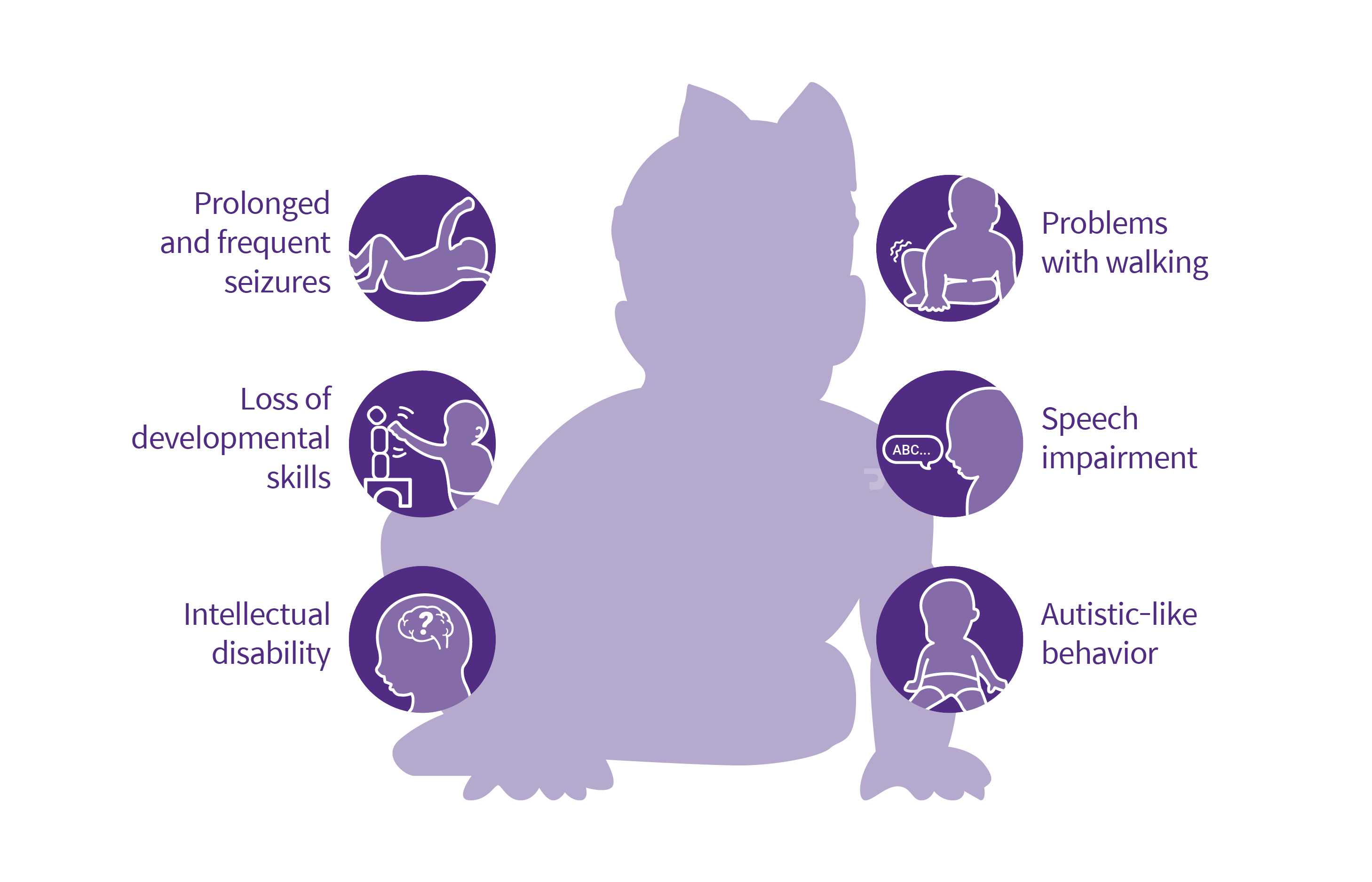
- Symptoms and characteristics: Dravet Syndrome is characterized by prolonged seizures, often triggered by fever (febrile seizures). Other seizure types, such as myoclonic seizures and generalized tonic-clonic seizures, may also occur. Developmental delays, cognitive impairment, and behavioral issues are common in children with Dravet Syndrome.
- Challenges in managing Dravet Syndrome: Similar to LGS, Dravet Syndrome is resistant to many anti-seizure medications. Seizures are often difficult to control, leading to significant challenges in managing the condition.
Introduction to Epidiolex
- Overview of Epidiolex as an FDA-approved medication: Epidiolex is the first FDA-approved prescription medication derived from CBD. It is specifically approved for the treatment of seizures associated with LGS and Dravet Syndrome in patients two years of age and older.
- Composition and mechanism of action: Epidiolex contains highly purified CBD derived from the Cannabis sativa plant. The exact mechanism of action is not fully understood, but CBD is believed to interact with various receptors in the brain involved in seizure activity.
- Legal status and availability: Epidiolex is classified as a Schedule V controlled substance, meaning it has a low potential for abuse. It is available by prescription in the United States and other countries where it has received regulatory approval.
Benefits of Epidiolex in LGS and Dravet Syndrome
- Reduction in seizure frequency: Clinical trials have shown that Epidiolex can significantly reduce the frequency of seizures in children with LGS and Dravet Syndrome compared to a placebo.
- Improvement in seizure control: Epidiolex has demonstrated effectiveness in improving seizure control, including a reduction in the number of drop seizures in patients with LGS.
- Potential cognitive and developmental benefits: Some studies suggest that Epidiolex may have positive effects on cognition and developmental outcomes in children with LGS and Dravet Syndrome, although further research is needed to fully understand these benefits.

Administration and Dosage
- Recommended dosage guidelines for LGS and Dravet Syndrome: The initial recommended dosage of Epidiolex for both LGS and Dravet Syndrome is 2.5 mg/kg twice daily. The dosage is gradually increased over several weeks based on individual response and tolerability, up to a maximum dosage of 10 mg/kg twice daily.
- Adjustments based on age, weight, and individual response: The dosage of Epidiolex may be adjusted based on the child’s age, weight, and individual response to treatment. Close monitoring by a healthcare professional is essential to determine the optimal dosage for each child.
- Titration process and dose escalation: Epidiolex is typically started at a low dose and gradually increased over several weeks. This titration process helps minimize potential side effects and allows the body to adjust to the medication.
Safety and Potential Side Effects
- Common side effects of Epidiolex: The most commonly reported side effects of Epidiolex include drowsiness, decreased appetite, diarrhea, and elevated liver enzymes. These side effects are generally mild to moderate in severity.
- Monitoring and management of side effects: Regular monitoring of liver function through blood tests is recommended during Epidiolex treatment. Healthcare professionals will closely monitor for any signs of liver abnormalities or other adverse effects.
- Potential drug interactions: Epidiolex may interact with other medications, especially those metabolized by liver enzymes. Healthcare professionals should be informed of all medications and supplements the child is taking to assess for potential drug interactions.
Considerations and Precautions
- Pregnancy and breastfeeding considerations: The use of Epidiolex during pregnancy or breastfeeding should be discussed with a healthcare professional. Limited data is available on its safety in these situations, and the potential risks and benefits need to be carefully considered.
- Driving and operating heavy machinery: Epidiolex can cause drowsiness, which may impair a child’s ability to drive or operate heavy machinery. It is important to monitor the child’s alertness and coordination while taking the medication.
- Other precautions and warnings: Children with a history of hypersensitivity to CBD or any of the components of Epidiolex should not use the medication. It is also important to inform healthcare providers about any pre-existing medical conditions, including liver problems.
Effectiveness and Long-Term Use
- Clinical studies supporting the effectiveness of Epidiolex: Clinical trials have shown that Epidiolex significantly reduces seizure frequency and improves seizure control in children with LGS and Dravet Syndrome compared to a placebo.
- Long-term use and maintenance therapy: Epidiolex is often used as a long-term maintenance therapy to manage seizures in children with LGS and Dravet Syndrome. The effectiveness and safety of long-term use are supported by ongoing studies and real-world clinical experience.
- Monitoring and follow-up care: Regular visits to a healthcare professional are essential to monitor the child’s response to Epidiolex, adjust the dosage if needed, and address any concerns or side effects. Ongoing communication and collaboration with healthcare providers are crucial for optimal management.
Patient and Caregiver Support
- Educational resources and support groups: Various educational resources and support groups are available to help patients and caregivers understand LGS, Dravet Syndrome, and the use of Epidiolex. These resources provide information, guidance, and a supportive community.
- Access to financial assistance programs: Financial assistance programs may be available to help eligible patients access Epidiolex. These programs can provide financial support or connect patients with resources to navigate insurance coverage or prescription assistance programs.
- Importance of open communication with healthcare providers: Maintaining open communication with healthcare providers is vital throughout the treatment journey. Regular updates on the child’s progress, any side effects, or concerns will aid in personalized care and adjustments to the treatment plan.
Conclusion
- Recap of key points: Epidiolex is an FDA-approved medication containing CBD, specifically indicated for the treatment of seizures associated with LGS and Dravet Syndrome in children.
- Future research and developments: Ongoing research aims to further understand the long-term effects, optimal dosages, and potential additional benefits of Epidiolex in LGS, Dravet Syndrome, and other seizure disorders.
- Encouragement for continued monitoring and treatment: Regular monitoring, follow-up care, and adherence to the treatment plan are crucial for managing seizures effectively in children with LGS and Dravet Syndrome.
Remember, it is crucial to consult with a healthcare professional for personalized guidance and recommendations tailored to the specific needs of the child with Lennox-Gastaut Syndrome or Dravet Syndrome.





